The Biological Imaging Facility is a core microscope imaging facility that specializes in widefield fluorescence, laser scanning confocal, spinning disk confocal, TIRF, and super-resolution microscopy (Lattice SIM, PALM, STORM), as well as traditional plant & animal microtechnique, histology, and cryotomy.
Facility Director: Denise Schichines, PhD & Facility Scientist: Jules Cho, PhD
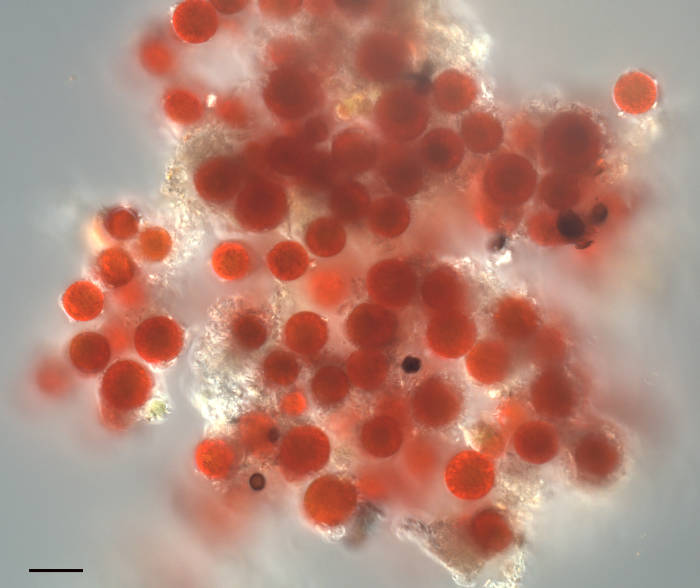
Image of “Watermelon Snow” or “Pink Snow” organism found in the Sierra Nevada mountains of California. The source of the pink color is a single-celled algae formerly called Chlamydomonas nivaloides, now renamed to Sanguina nivaloides. This genus contains two species: S. nivaloides which makes pink snow, and S. aurantia which makes orange snow. These algae produce a carotenoid (astaxanthin) that protects its photosynthetic pigments from UV damage. The algae are dormant during the summer and snow-covered winter months. During the Spring however, the snow melts creating liquid water. This stimulates germination producing green flagellated cells that swim to the surface. Here the cells shed the flagella to form these photosynthetic cells, or cysts. Each Sanguina cyst contains a single chloroplast surrounding a nucleus, with the remaining cytoplasm filled with red astaxanthin. No culturable strains exist, thus the vegetative and reproductive stages are unknown. Sanguina nivaloides is referred to as a cryophillic/extremophillic algae as it is found only in alpine snowfields. This sample was collected above 9000′ near Mammoth Mountain, CA. Scalebar = 20µm.
Steve Ruzin, curator of the Golub Antique Microscope Collection, UCB
The Rausser College of Natural Resource’s Biological Imaging Facility functions as an instructional and research laboratory for all aspects of modern light microscopy, including confocal and super-resolution microscopy, image processing and analysis, and most microscopical techniques for developmental and cell biology. Computer image processing and analysis is taught on an individual basis. Microscopy is taught on two levels:
- Individually as needed
- PMB185 Techniques in Light Microscopy (this class has been discontinued as of Fall 2024)
In addition, the Facility offers a one-week workshop in Plant & Animal Microtechnique to train the student in modern and classical methods in making microscope slide preparations. Please contact us for more details on our next workshop.
There are four core laboratories on campus that offer expertise, instruction, and instrumentation in microscopy for research.
- The CNR Biological Imaging Facility (This lab): widefield, confocal, and super-resolution epifluorescence microscopy, live-cell imaging, microtechnique, training in digital image processing and analysis. Located in 381 Koshland Hall
- Molecular Imaging Center: Two-photon, SPIM, confocal microscopy, electrophysiology, live-cell imaging, and FLIM. Located in Weill, Li Ka Shing, and Barker Halls.
- Electron Microscope Laboratory: Transmission and scanning electron microscopy. Located in Barker Hall.
- CIRM/QB3 Shared Stem Cell Facility: Available for the study of stem cells. Located in Stanley Hall
- The Golub Microscope Collection: Web site dedicated to Dr. Orville J. Golub’s collection of antique microscopes from the 17th–20th Centuries. The collection is located in VLSB. Dr. Steve Ruzin is the Curator.