|
Technique
|
Basis
|
Components
|
When Used
|
Example
|
| WF Fluorescence | Excitation of intrinsic or applied fluorophore visualized through discriminating filters localize fluorescence emitting probes on a dark background.
WF microscope accepts all emitted light whether in- or out-of-focus, thus images can be blurry. |
1) Hi-Intensity light source (Hg, Xe) 2) Excitation filter passes only Ex wavelength 3) Dichroic beamsplitter reflects Ex light, transmits Em light 4) Emission filter passes Em light, rejects scattered (Ex) light |
General purpose optical method for visualizing fluorescent probe molecules. Depends on a) specificity of probe, and b) specificity of fluorophore wavelength. Resolution is diffraction limited (250nm) | 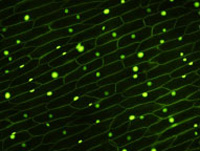 Onion nuclei localized with SYTO |
| Laser Scanning Confocal | Excitation of intrinsic or applied fluorophore visualized through discriminating filters or spectral arrays localize light emitting probes on a dark background.
Confocal imaging rejects out-of-focus Em light by interposing a (variable diameter) pinhole between the sample and detector at a plane conjugate to the sample focal plane. |
1) Laser excitation light source scans the sample in a raster (Z) pattern 2) Dichroic beamsplitter Reflects Ex light, Transmits Em light (beamsplitter is usually glass but may be an AOTF) 3) Em filter passes sample fluorescence 4) Pinhole conjugate to the sample plane passes only in-focus light 5) PMT detectors record fluorescence intensity |
Same as WF, except used when optical sectioning is required for 3D analysis and/or reconstruction.
Useful for relatively thin samples (<200µm) Laser excitation can cause photobleaching. Resolution is slightly better due to pinhole effects (200nm) |
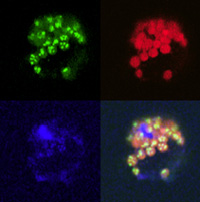 Plant protoplast showing three different probes in chloroplasts. 4th is the composite. |
| 2-Photon imaging | Excitation of intrinsic or applied fluorophore visualized through discriminating filters or spectral arrays localize light emitting probes on a dark background.
2-photon effect excites fluorophore only at objective focal point. No out-of-focus fluorescence is generated. |
1) IR Pulse Laser excitation light source generates 2P effect at the focal point of the objective 2) Dichroic beamsplitter Reflects Ex light, Transmits Em light (beamsplitter may be an AOTF) 3) There is NO pinhole 4) Non-Descanned (PMT) detectors record fluorescence intensity. |
Same as WF, except used when optical sectioning is required for 3D analysis and/or reconstruction.
Useful for very thick tissues (<500µm), as IR penetrates further than visible Ex light. IR "burning" of the sample can be a problem. Lower resolution than confocal imaging (resolution a function of Ex wavelength); usually about 300nm in X &Y; but comparable to confocal due to the extremely small 2P fluorescence spot. (i.e., smaller than Abbe would predict.) |
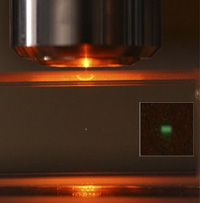 2P Fluorescence at the focal point. |
| Restoration ("Deconvolution") | Excitation of intrinsic or applied fluorophore visualized through discriminating filters localize light emitting probes on a dark background.
A WF technique that acquires all Em light in all focal planes. Computational methods ("deconvolution") restore Z-position of voxel intensity values. |
[Same as WF fluorescence]: 1) Hi-Intensity light source 2) Excitation filter passes only Ex wavelength 3) Dichroic beamsplitter Reflects Ex light, Transmits Em light 4) Emission filter passes Em light, rejects scattered light 5) Requires the PSF of the optical system to correctly deconvolve image stack. |
Very good for imaging small, dimly fluorescent samples (<5µm). High-sensitivity CCD detectors allow low fluorescence intensities to decrease photobleaching.
Requires High-NA objectives. Resolution can be 200nm or slightly better. |
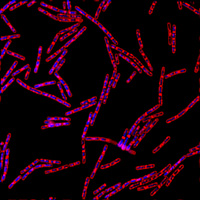 Bacillus subtilis cells probed with DAPI (DNA) and FM4-64 (outer membrane). |
| Spinning Disk Confocal | Excitation of intrinsic or applied fluorophore visualized through discriminating filters or spectral arrays localize light emitting probes on a dark background.
Confocal imaging rejects out-of-focus Em light via a (fixed diameter) pinhole(s). |
[Same as WF fluorescence]: 1) Hi-Intensity light source (Hg or Laser) 2) Excitation filter passes only Ex wavelength 3) Dichroic beamsplitter Reflects Ex light, Transmits Em light 4) Emission filter passes Em light, rejects scattered light Spiral array of pinholes on a spinning disk creates an array of Ex point sources that sweep across the sample. Em light is descanned by the same pinhole array and imaged on a CCD camera. |
Same as WF, except used when optical sectioning is required for 3D analysis and/or reconstruction.
Useful for relatively thin samples (<200µm). Fixed pinhole diameter normalized for a 63x objective a) decreases efficiency of out-of-focus light rejection; b) reduces lateral and axial resolution; usually worse than 250nm. Ex light throughput increased to >70% using microlenses over each pinhole. Sweeping array of Ex light provides rapid confocal imaging for dynamic live-cell microscopy. |
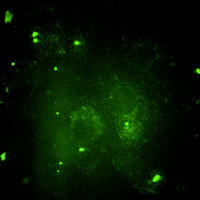 Living macrophage expressing GFP. |
| SPIM | AKA: Lightsheet imaging
WF fluorescence but with the transparent sample illuminated by a thin plane ("sheet") of Ex light. Resolution is limited to the thickness of the sheet (usually 1µm). |
Microscope with the Illuminating objective set 90° from the imaging objective. Requires the sample to be completely transparent ("Clarity"). Each image is a full cross-section of the sample. | Useful for large, cleared samples (e.g., entire mouse brain or embryo).
A High-Res modification is the Lattace Lightsheet invented by Betzig. Its sheet is thin by wave interference ("Bessel Beam") |
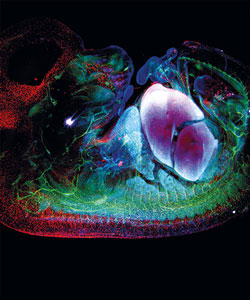 |