|
Technique
|
Basis
|
Components
|
When Used
|
Example
|
| Total Internal Reflection (TIRF) | Excitation of intrinsic or applied fluorophore visualized through discriminating filters or spectral arrays localize light emitting probes on a dark background.
Ex source is a laser impinging on the sample at (or greater than) the critical angle, resulting in no propagation of Ex light into sample. Evanescent energy wave, however, can excite fluorophores in sample, but only within 100nm (or so) from the inner (sample side) surface of the coverslip. |
Standard WF fluorescence microscope with very high NA objective.
Ex source is a laser. Detector usually a high-sensitivity CCD camera (EMCCD) |
Technique is very good for visualizing fluorescent probes on the surface of the sample (e.g., plasma membrane). Excites fluorophores only within 100nm of the coverslip surface. | 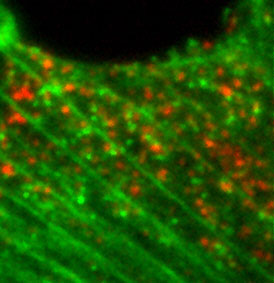 Endocytosis of probe molecules (red) just inside the PM. |
| Fluorescence Recovery After Photobleaching (FRAP) |
A WF or Confocal fluorescence technique for living cells. A sample ROI is purposely photobleached (often by a laser of a wavelength different from the fluorophore Ex), then the restoration of fluorescence is followed either qualitatively (visually) or quantitatively. | Same as Laser Scanning Confocal, but with ROI scanning capability for selective photobleaching. Most Laser Scanning Confocal microscopes can do this technique. WF systems require a separate photobleaching "module". | A live-cell technique used to study cell dynamics (movement) or diffusion of fluorescenated molecules. | 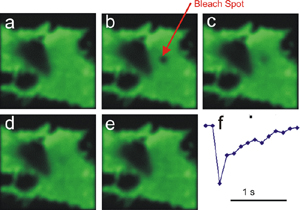 |
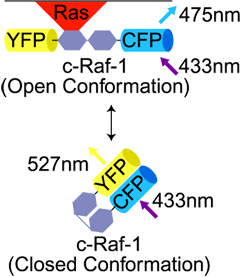
| Förster Resonance Energy Transfer (FRET)
Often called Fluorescence Resonance Energy Transfer |
A WF or Confocal fluorescence technique using a pair of fluorescent molecules. The excitation wavelength is selected for the Donor ("1st") molecule. The emission wavelength of the Acceptor ("2nd") molecule is detected.
FRET occurs only if Donor and Acceptor molecules are <10nm apart. |
Standard WF or Confocal microscope.
The critical components in this technique are the Donor/Acceptor pair of probes. Example: |
A technique used to determine if two molecules are close together (<10nm). e.g., signaling molecule and its receptor molecule.
Can look for either: 1) Emission of Acceptor fluorescence after Donor Ex |
 |
| Fluorescence Lifetime Imaging (FLIM) | A WF, Confocal, or 2P technique that measures the length of time a population of fluorescent molecules fluoresces after the initial excitation event. Fluorescence decay is measured in nsec; typically 50ns. | No changes to optical components. High-speed electronics and camera required. Ex source a pulse laser. |
A technique that determines precise kinetics (and localization) of fluorophores, often in living systems. An alternate detection method for determining FRET. Imaging a sample takes a long time (maybe 30 minutes) |
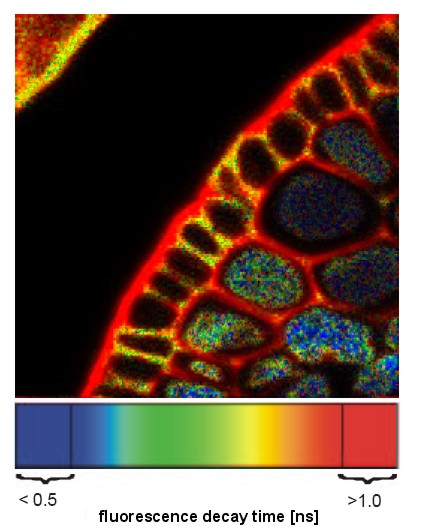 Image of plant root XS pseudocolored showing varying fluorescence lifetime in different compartments. |
| Stimulated Emission Depletion (STED) | A confocal technique that uses an axial "depletion" laser of a long wavelength (594nm) to stimulate the emisison of the fluorophore of interest (usually GFP) to red instead of green. The depletion laser is toroidal in section. This produces a red doughnut with a small green excitation spot in the middle. The green spot is sub-resolution. | Normal confocal microscope Normal excitation laser (blue) High-Power red depletion laser. |
When super-resolution is required (<<200nm). 50nm
Fails in many samples due to high stimulated background emission. |
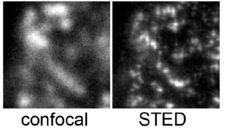 |
| Photoactivated Localization Microscopy (PALM)
STORM: Stochastic Optical Reconstruction Microscopy |
A WF technique that relies on the stochastic (random) "activation" (emission) of specialized fluorophores (e.g., PA-GFP or certain Cyanine dyes). An excitation laser induces fluorescence emission of a small subset of single fluorophores while the majority remain "dark". Analysis then calculates the center of the fluorescence, which is interpreted as the center of the Airy disc. Software records this as the position of the single molecule. | Standard WF fluorescence microscope with high-NA objective. Ex laser Computer to determine the computed center of the Airy disc |
Use when super-resolution is required (<<200nm). 20nm can be achieved
PALM only work with photo-activatible (PA) fluorophores. STORM works with Cyanine dyes and the ability to keep them in a hyper-activated state |
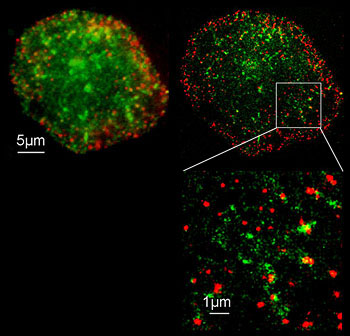 |
| Structured Illumination Microscopy (SIM) | A WF technique that superimposes a known optical pattern over the fluorescence of the sample. Subsequent analysis of the resolvable Móire pattern (resulting from the interaction of sample fluorescence and the excitation pattern) reveals subresolution structure within the sample. | Standard WF fluorescence microscope, Super-stable optical table, Low-light digital camera(s), Computer for computational analysis of Móire patterns. | Use when super-resolution is required. Can achieve 100nm structures in X,Y, and Z. Fails if there is movement of sample. |
 |