Förster Resonance Energy Transfer (FRET) is a physical phenomenon whereby energy created by fluorescence excitation of one molecule is transferred to an adjacent molecule. This is also called Fluorescence Resonance Energy Transfer. If the two molecules are fluorophores, then excitation of the first molecule (Donor) results in fluorescence emission of the second molecule (Acceptor).

In this diagram (from here) the donor molecule is excited by a photon within its excitation spectrum. Under typical fluorescence conditions relaxation of the electron energy would result in the emission of a photon (fluorescence) by the donor fluorophore, with a resultant drop in molecule energy (down arrows). However, if a suitable acceptor molecule is within a certain distance (Förster distance; <10nm) then donor energy can experience transfer of energy (Kr) to the Acceptor, resulting in Acceptor fluorescence. An important concept is that the transfer of energy between molecules is nonradiative, and that the distances involved are much less than the wavelength of light. Föster energy transfer is therefore distinguished from a more common radiative transfer of energy (e.g., absorption of the Ex photon by a molecule.)
In a FRET-pair system, Donor Excitation results in Acceptor Emission. It also results in changes in
Fluorescence Lifetime.
Fluorescence Lifetime.

Requirements for FRET:
- Molecules must be <10nm apart.
- Donor emission must overlap Acceptor excitation
A common example of a FRET-Pair is CFP/YFP (left).
What does FRET tell us:
- Molecular interactions
- Ligand binding to a receptor
- Protein-Protein interactions
- Molecular dimerization
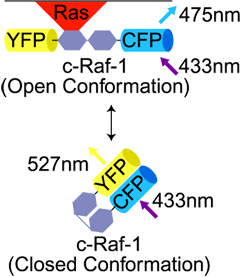
Here is a biological example where FRET was used to determine the conformation of a signaling molecule c-Raf. By adding the FRET pairs CFP and YFP to the ends of c-Raf, the researchers were able to distinguish the open conformation (no FRET) from the closed conformation (FRET fluorescence = YFP emission). The movie shows (in grayscale and color) the transformation from Open (CFP emission, blue) to Closed conformation (YFP emission, yellow) over time.
Analogs to FRET are:
- BRET, Bioluminescence Resonance Energy Transfer, where the donor emitts light through luminescence (typically Luciferase).
- Acceptor Photobleaching: where the rate of photobleaching of the Acceptor molecule is measured with and without Donor. Here increased Donor photobleaching resistance (longer fluorescence before bleaching) implys Donor/Acceptor FRET.