Fluorescence
In biology, fluorescence microscopy is used most often as a method of visualizing a probe molecule within a sample. Unlike brightfield microscopy, where contrast is a result of light absorption, fluorescence microscopy generates contrast by emission of light. That is, the molecule that we are interested in seeing (the Probe) glows a known color, and the background emits no color. (Under ideal conditions, the background will be completely dark.)
Fluorescence: The absorption of light of a particular span of wavelengths (color) by a molecule, and the re-irradiation of light of longer wavelengths (lower energy) and, of course, a different color. If the time between absorption and re-emission is < 10e-5 to 10e-8sec, then this phenomenon is called Fluorescence (F). If the time is longer (often much longer) then the phenomenon is called Phosphorescence (P).
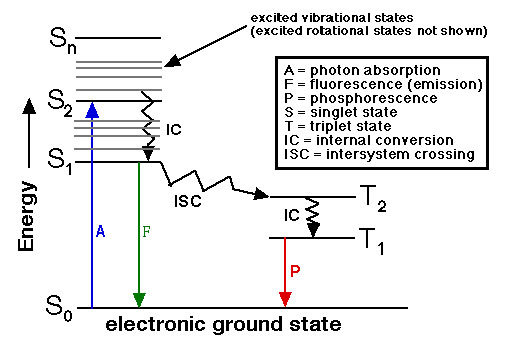
Energy states of electrons are quantized into distinct "levels" with S0 being the low energy Ground State. When an electron absorbs electromagnetic energy (in the form of a photon) the energy of the electron increases to higher states (S1, S2, etc.) There are two general Transition methods by which the energy of the electron diminishes: Radiative and non-radiative.
Radiative: Fluorescence and Phosphorescence
Non-radiative: The most important to microscopists is intersystem crossing (ISC). Here the energy is transferred to another molecule by physical collision. An important example of this is the mechanism by which Anti-Fade compounds work. It is also the basis for FRET Pair interaction.
Above is a graphical representation of energy transfer within molecules is called a Jablonski Diagram (named after the Polish physicist Aleksander Jablonski 1898–1980). Radiative energy transitions are represented as solid arrows. Non-radiative transitions are shown as zigzagged lines. Fluorescence thus is a molecular mechanism that dissipates energy in the form of photons of light. This phenomenon is used as a Optical Reporter. Examples where fluorescence is used are: Fluorescent lighting, LEDs, mineralogy, and fluorescence microscopy.
The efficiency of fluorescence is called Fluorescence Quantum Yield